19 magnificent images from the Astronomy Photographer of the Year shortlist
A galactic worm gobbles stars. A plasma whale slides across the sun‘s surface. And an eerie dragon dances with an aurora. It’s not the plot to a fantasy novel, it’s our incredible universe captured in stunning detail.
The Royal Observatory Greenwich has announced the shortlisted images for the 2024 Astronomy Photographer of the Year. The finalists were selected from more than 3,500 images submitted from professional and amateur photographers from 58 countries. The winner will be announced September 12 and an exhibition of the top images will be on display in London at the National Maritime Museum starting September 14.

Gwenaël Blanck travelled to Australia in April 2023 to see the 62-second long total solar eclipse. In this collage he shows the corona and the pink chromosphere, the prominences and Baily’s beads, chinks of sunlight that shine through due to the Moon’s rugged landscape. The image is made of seven superimposed pictures, one overexposed for the background and six others for the chromosphere and prominences. Image: © Gwenaël Blanck (France)

This photograph of the Geminid meteor shower was taken under perfect conditions on La Palma. During the peak of the night, Sahner could easily spot two or three or more meteors per minute within the field of view. The panorama shows the entire winter Milky Way as seen from La Palma in RGB natural colour with extra details in H-alpha. Image: © Jakob Sahner (Germany)

M81, also known as Bode’s Galaxy, is about 11.75 million light years away in the constellation Ursa Major. It is one of the brighter galaxies in the night sky. In the image’s background, some Integrated Flux Nebula (IFN) can be seen. IFN is dust outside the Milky Way’s galactic plane that is illuminated only by the stars in the Milky Way. Image: © Holden Aimar (USA), aged 14

A view of the Eystrahorn Mountain (Iceland) on the night of a KP7 storm (a strong geomagnetic storm that can cause aurorae and upset electrical power systems). The intensity of the storm resulted in the impressive range of colours in the sky. Image: © Jose Miguel Picon Chimelis (Spain) JOSE CHIMELIS

The photographer was able to capture the aurora in motion when it turned into something resembling a dragon’s head on a clear night. Telser chose to use black and white to emphasise the contrast of the aurora against the dark sky. Image: © Moritz Telser (Italy) MORITZ TELSER

This image shows the details of the Sun’s surface. The photographer views the shape of the filament to the left of the disc as an immense plasma whale traversing the solar surface. Eduardo Schaberger Poupeau captured this photo by recording two videos (one for the disc and another for the prominences), each consisting of 850 frames. Image: © Eduardo Schaberger Poupeau (Argentina)

This image shows an abandoned house in the middle of the Namib Desert with the Milky Way rising above it. The sky was captured with a star tracker to lower the ISO. The veil of clouds and halos around the stars create a dreamlike effect. Image: © Stefan Liebermann (Germany)

This impressive aurora, which seemingly takes the form of a dragon, was the result of a geomagnetic storm (level G2) generated by a coronal mass ejection. The photo was captured at the Arctic Henge, which was one of the only places in Iceland with clear skies that night. Image: © Carina Letelier Baeza (Chile) Cari Letelier

This image captures the International Space Station (ISS) in transit across October’s Full Moon, the Hunter’s Moon, approximately 12 hours after a partial lunar eclipse. The striking beauty of the Full Moon is on display, with its mix of rugged highlands, bright crater rays and darker maria. Image: © Tom Glenn (USA)

Mount Aso in Kumamoto Prefecture is the collective name for the five peaks often called the ‘Five Mountains of Aso’. One of the peaks, Nakadake, has a volcanic crater that is still active. Abe wanted this image to show how the Milky Way has watched over activity on Earth since prehistoric times. This is a composite photograph with the foreground and sky photographed separately but without moving the tripod. Image: © Yoshiki Abe (Japan)

In this image the photographer was able to capture a dwarf planet, Ceres, more than a billion times smaller than its galactic counterpart, transit beyond the galaxy’s spiralling arms. Ceres shines brighter than the galaxy and moves quickly across the night sky. For this image, multiple long exposures were captured over an eight-hour period to showcase the beauty of the Blowdryer Galaxy and the relatively quick speed of the dwarf planet Ceres. Image: © Damon Mitchell Scotting (UK)

CG4 (Cometary Globule 4) is a complex of nebulosity and dust with a very peculiar shape, located in the southern constellation of Puppis. The ‘head’ of the galactic worm has dimensions of about 1.5 light years. This image is the result of the work of a team of astrophotographers: they joined forces to rent the powerful Newtonian 500-mm telescope from Chilescope service, processing the raw files and then voted for the best images. Image: © ShaRa

This image was taken at Snettisham Beach, famous for its vast tidal mudflats that attract migrating birds in staggering numbers. The foreground subject is a dilapidated jetty, which was built in the Second World War to allow gravel extracted from the nearby pits to be moved by boat. The curved channel in the mudflat mirrors the trailing stars. Image: © Paul Haworth (UK)

This photograph captures a sculpture in north-west Namibia. Made of stone, this is one of a group of sculptures known as the ‘Lone Men of Kaokoland’ [as the region was formerly known]. No one knows who has put them there. A long exposure of the stone running man was taken first, then the tripod was moved for a clear view of the horizon. Image: © Vikas Chander (India)

During the Spring Festival, the Sun and altostratus clouds acted together to create this huge corona, soaring above the Himalayas. The result is an enormous colour palette above the snowy peaks. Image: © Geshuang Chen (China)

Saturn’s decreasing ring tilt means the moon Titan is closer to Saturn from our viewpoint than it has been in over a decade. At the centre of the image, Tethys is just about to disappear behind Saturn, while Rhea, Enceladus and Mimas are on the left, and Dione is to the lower right. The planet’s shadow on the rings is prominent, as are the Cassini and Encke divisions. Image: © Andy Casely (Australia)

The intense brightness of M104’s core often hides the details that lie inside the encircling ring of dust. In this image the dust appears to spiral into that core, floating on a wafer-thin layer as it falls towards the massive central black hole. The brighter, more colourful stars in the image are actually in the foreground − a part of our Milky Way galaxy. Image: © Kevin Morefield (USA)
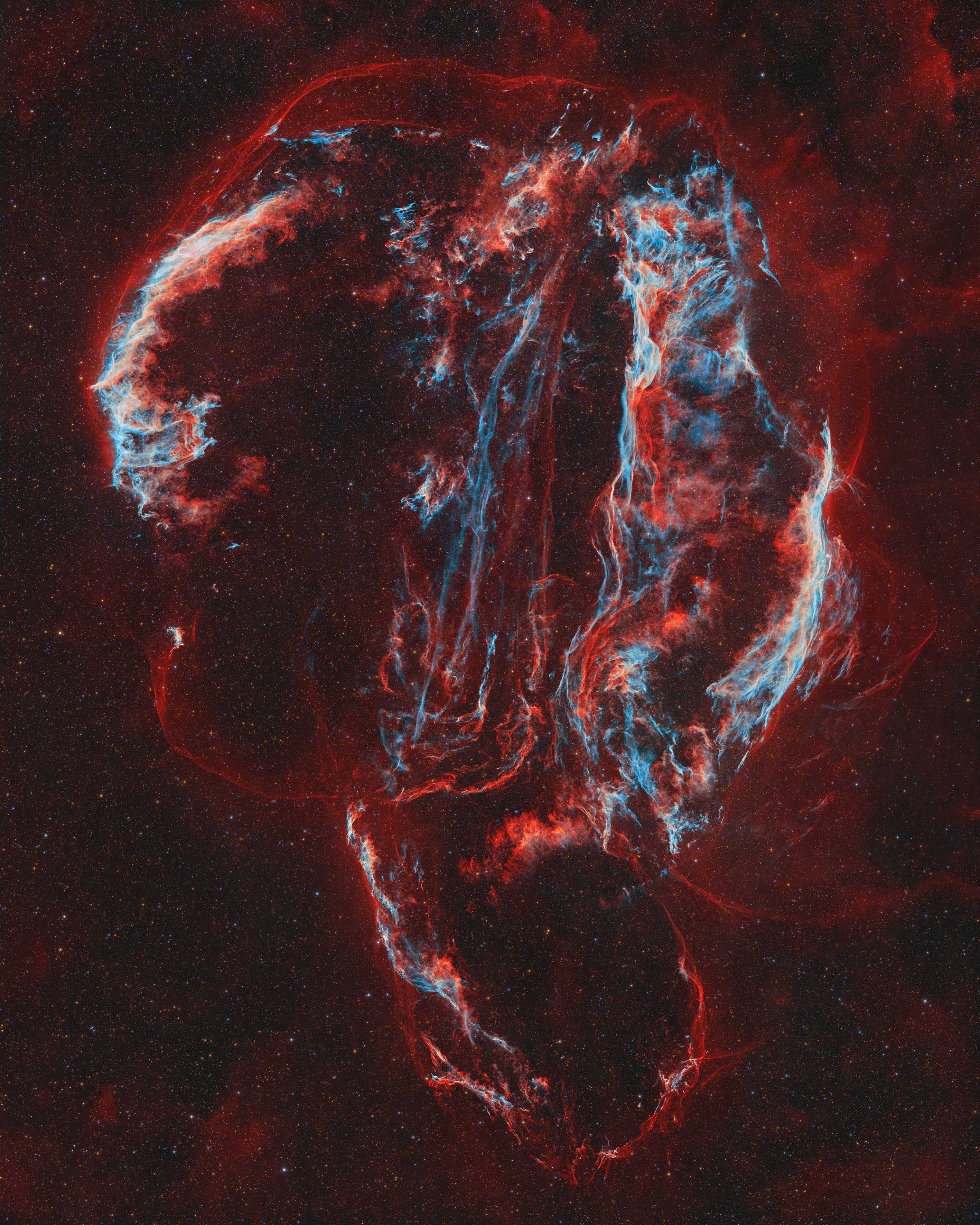
The Cygnus supernova afterglow is a popular object with astrophotographers, but the idea here was to take advantage of the high quality of the sky and the long exposure time to highlight details that are rarely seen, such as the outer envelope of the supernova remnant. The image’s name is a nod to The Scream, the famous painting by Edvard Munch, symbolising the scream that continues to echo through space after the star’s death. Image: © Yann Sainty (France)
The post 19 magnificent images from the Astronomy Photographer of the Year shortlist appeared first on Popular Science.