MIT researchers discover “neutronic molecules”
Neutrons are subatomic particles that have no electric charge, unlike protons and electrons. That means that while the electromagnetic force is responsible for most of the interactions between radiation and materials, neutrons are essentially immune to that force.
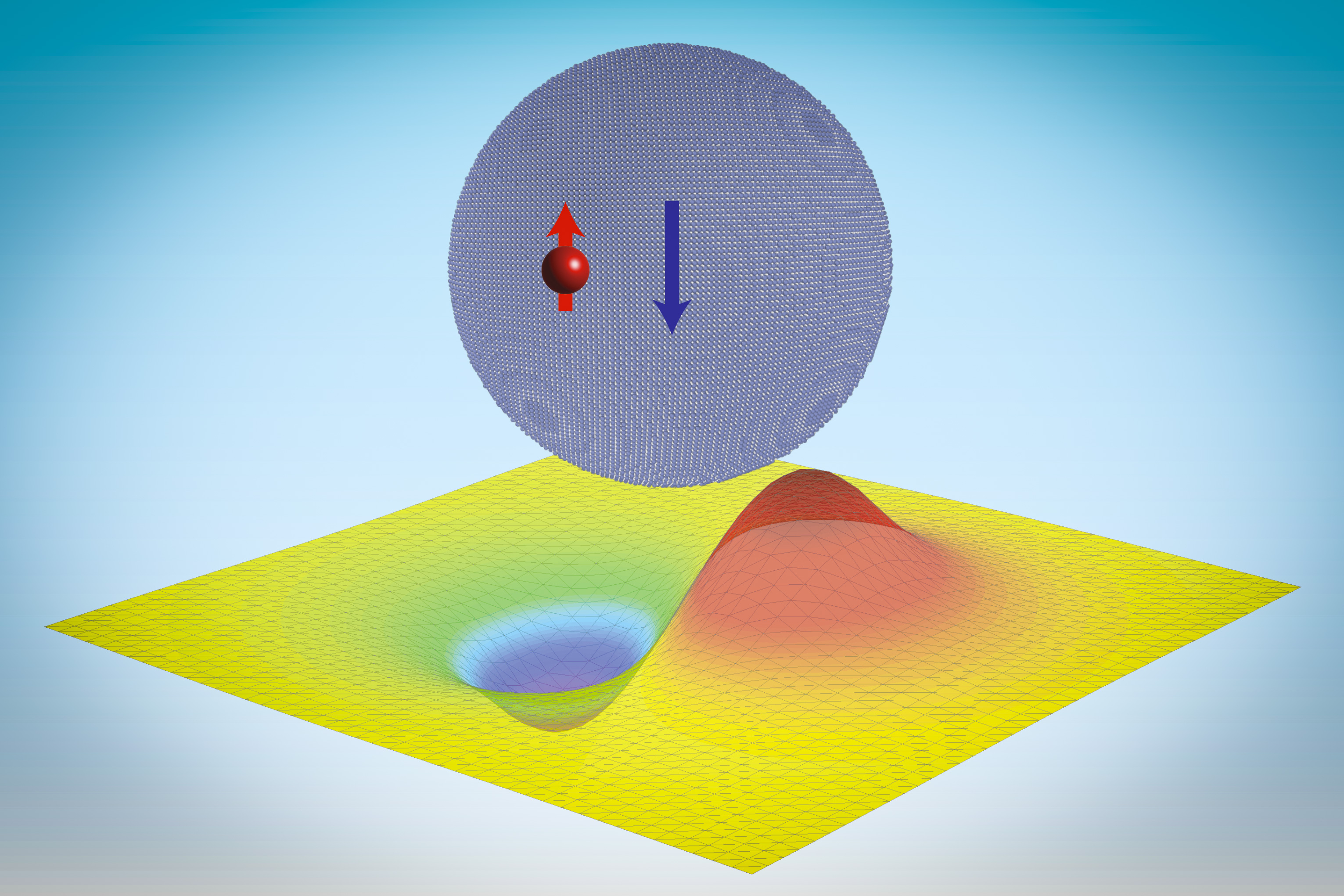
Instead, neutrons are held together inside an atom’s nucleus solely by something called the strong force, one of the four fundamental forces of nature. As its name implies, the force is indeed very strong, but only at very close range — it drops off so rapidly as to be negligible beyond 1/10,000 the size of an atom. But now, researchers at MIT have found that neutrons can actually be made to cling to particles called quantum dots, which are made up of tens of thousands of atomic nuclei, held there just by the strong force.
The new finding may lead to useful new tools for probing the basic properties of materials at the quantum level, including those arising from the strong force, as well as exploring new kinds of quantum information processing devices. The work is reported this week in the journal ACS Nano, in a paper by MIT graduate students Hao Tang and Guoqing Wang and MIT professors Ju Li and Paola Cappellaro of the Department of Nuclear Science and Engineering.
Neutrons are widely used to probe material properties using a method called neutron scattering, in which a beam of neutrons is focused on a sample, and the neutrons that bounce off the material’s atoms can be detected to reveal the material’s internal structure and dynamics.
But until this new work, nobody thought that these neutrons might actually stick to the materials they were probing. “The fact that [the neutrons] can be trapped by the materials, nobody seems to know about that,” says Li, who is also a professor of materials science and engineering. “We were surprised that this exists, and that nobody had talked about it before, among the experts we had checked with,” he says.
The reason this new finding is so surprising, Li explains, is because neutrons don’t interact with electromagnetic forces. Of the four fundamental forces, gravity and the weak force “are generally not important for materials,” he says. “Pretty much everything is electromagnetic interaction, but in this case, since the neutron doesn’t have a charge, the interaction here is through the strong interaction, and we know that is very short-range. It is effective at a range of 10 to the minus 15 power,” or one quadrillionth, of a meter.
“It’s very small, but it’s very intense,” he says of this force that holds the nuclei of atoms together. “But what’s interesting is we’ve got these many thousands of nuclei in this neutronic quantum dot, and that’s able to stabilize these bound states, which have much more diffuse wavefunctions at tens of nanometers [billionths of a meter]. These neutronic bound states in a quantum dot are actually quite akin to Thomson’s plum pudding model of an atom, after his discovery of the electron.”
It was so unexpected, Li calls it “a pretty crazy solution to a quantum mechanical problem.” The team calls the newly discovered state an artificial “neutronic molecule.”
These neutronic molecules are made from quantum dots, which are tiny crystalline particles, collections of atoms so small that their properties are governed more by the exact size and shape of the particles than by their composition. The discovery and controlled production of quantum dots were the subject of the 2023 Nobel Prize in Chemistry, awarded to MIT Professor Moungi Bawendi and two others.
“In conventional quantum dots, an electron is trapped by the electromagnetic potential created by a macroscopic number of atoms, thus its wavefunction extends to about 10 nanometers, much larger than a typical atomic radius,” says Cappellaro. “Similarly, in these nucleonic quantum dots, a single neutron can be trapped by a nanocrystal, with a size well beyond the range of the nuclear force, and display similar quantized energies.” While these energy jumps give quantum dots their colors, the neutronic quantum dots could be used for storing quantum information.
This work is based on theoretical calculations and computational simulations. “We did it analytically in two different ways, and eventually also verified it numerically,” Li says. Although the effect had never been described before, he says, in principle there’s no reason it couldn’t have been found much sooner: “Conceptually, people should have already thought about it,” he says, but as far as the team has been able to determine, nobody did.
Part of the difficulty in doing the computations is the very different scales involved: The binding energy of a neutron to the quantum dots they were attaching to is about one-trillionth that of previously known conditions where the neutron is bound to a small group of nucleons. For this work, the team used an analytical tool called Green’s function to demonstrate that the strong force was sufficient to capture neutrons with a quantum dot with a minimum radius of 13 nanometers.
Then, the researchers did detailed simulations of specific cases, such as the use of a lithium hydride nanocrystal, a material being studied as a possible storage medium for hydrogen. They showed that the binding energy of the neutrons to the nanocrystal is dependent on the exact dimensions and shape of the crystal, as well as the nuclear spin polarizations of the nuclei compared to that of the neutron. They also calculated similar effects for thin films and wires of the material as opposed to particles.
But Li says that actually creating such neutronic molecules in the lab, which among other things requires specialized equipment to maintain temperatures in the range of a few thousandths of a Kelvin above absolute zero, is something that other researchers with the appropriate expertise will have to undertake.
Li notes that “artificial atoms” made up of assemblages of atoms that share properties and can behave in many ways like a single atom have been used to probe many properties of real atoms. Similarly, he says, these artificial molecules provide “an interesting model system” that might be used to study “interesting quantum mechanical problems that one can think about,” such as whether these neutronic molecules will have a shell structure that mimics the electron shell structure of atoms.
“One possible application,” he says, “is maybe we can precisely control the neutron state. By changing the way the quantum dot oscillates, maybe we can shoot the neutron off in a particular direction.” Neutrons are powerful tools for such things as triggering both fission and fusion reactions, but so far it has been difficult to control individual neutrons. These new bound states could provide much greater degrees of control over individual neutrons, which could play a role in the development of new quantum information systems, he says.
“One idea is to use it to manipulate the neutron, and then the neutron will be able to affect other nuclear spins,” Li says. In that sense, he says, the neutronic molecule could serve as a mediator between the nuclear spins of separate nuclei — and this nuclear spin is a property that is already being used as a basic storage unit, or qubit, in developing quantum computer systems.
“The nuclear spin is like a stationary qubit, and the neutron is like a flying qubit,” he says. “That’s one potential application.” He adds that this is “quite different from electromagnetics-based quantum information processing, which is so far the dominant paradigm. So, regardless of whether it’s superconducting qubits or it’s trapped ions or nitrogen vacancy centers, most of these are based on electromagnetic interactions.” In this new system, instead, “we have neutrons and nuclear spin. We’re just starting to explore what we can do with it now.”
Another possible application, he says, is for a kind of imaging, using neutral activation analysis. “Neutron imaging complements X-ray imaging because neutrons are much more strongly interacting with light elements,” Li says. It can also be used for materials analysis, which can provide information not only about elemental composition but even about the different isotopes of those elements. “A lot of the chemical imaging and spectroscopy doesn’t tell us about the isotopes,” whereas the neutron-based method could do so, he says.
The research was supported by the U.S. Office of Naval Research.
© Image: Courtesy of the researchers


 Scientists tested delicate Bose-Einstein condensates in their instruments, which could one day undergird ultrasensitive accelerometers.Qinetiq
Scientists tested delicate Bose-Einstein condensates in their instruments, which could one day undergird ultrasensitive accelerometers.Qinetiq
